A) mg = tetrahedral
B) mg = trigonal pyramidal
C) mg = trigonal planar
D) mg = trigonal bipyramidal
E) mg = bent
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules have sp3d hybridization on the central atom? SiCl4 BrF5 AsF5 BrF3
A) 2
B) 0
C) 4
D) 1
E) 3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules are polar? BrCl3 CS2 SiF4 SO3
A) 1
B) 2
C) 3
D) 4
E) 0
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule containing a central atom with six electron groups has a(n) ________ electron geometry.
A) octahedral
B) trigonal bipyramidal
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for BrO4⁻. What is the hybridization on the Br atom?
A) sp
B) sp3d2
C) sp3d
D) sp3
E) sp2
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry for the molecule PCl5.
A) Linear
B) Trigonal planar
C) Tetrahedral
D) Trigonal bipyramidal
E) Octahedral
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule containing a central atom with sp3d hybridization has a(n) ________ electron geometry.
A) tetrahedral
B) linear
C) octahedral
D) trigonal planar
E) trigonal bipyramidal
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for BF3. What is the hybridization on the B atom?
A) sp
B) sp3d2
C) sp3d
D) sp3
E) sp2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is paramagnetic. 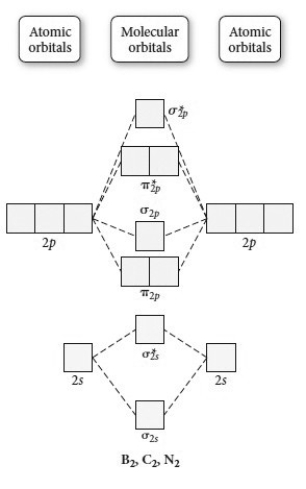
A) B22⁺
B) B22⁻
C) N22⁺
D) C22⁻
E) B2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of IBr4-.
A) eg = tetrahedral, mg = tetrahedral
B) eg = octahedral, mg = square planar
C) eg = trigonal planar, mg = bent
D) eg = tetrahedral, mg = trigonal pyramidal
E) eg = trigonal bipyramidal, mg = linear
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of the bolded and underlined atom CH3OCH3.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = linear
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
List the number of sigma bonds and pi bonds in a double bond.
A) 1 sigma, 1 pi
B) 0 sigma, 2 pi
C) 2 sigma, 0 pi
D) 1 sigma, 2 pi
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the molecular geometry for the molecule PCl5.
A) Linear
B) Seesaw
C) Square pyramidal
D) Trigonal bipyramidal
E) Square planar
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -sp
A) sp hybridized central atom
B) octahedral electron geometry
C) polar, but contains no polar bonds
D) sp2 hybridized central atom
E) linear
F) nonpolar, but contains a polar covalent bond
G) tetrahedral
H) trigonal planar
I) octahedral
J) polar
K) trigonal bipyramidal
L) seesaw molecular geometry
N) I) and J)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of XeF2.
A) eg = trigonal bipyramidal, mg = bent
B) eg = linear, mg = linear
C) eg = tetrahedral, mg = linear
D) eg = trigonal bipyramidal, mg = linear
E) eg = tetrahedral, mg = bent
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model, the molecular geometry of the central atom in Se O2 is ________.
A) linear
B) tetrahedral
C) bent
D) trigonal planar
E) trigonal pyramidal
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model, the molecular geometry of the central atom in CCl4 is ________.
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for OF2. What is the hybridization on the O atom?
A) sp
B) sp3
C) sp2
D) sp3d
E) sp3d2
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for the molecule CH3CH2CCH. How many sigma and pi bonds does it contain?
A) 11 sigma, 0 pi
B) 9 sigma, 1 pi
C) 8 sigma, 3 pi
D) 9 sigma, 2 pi
E) 8 sigma, 2 pi
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules have sp3d2 hybridization on the central atom? SeCl6 XeF4 IF5 AsCl5
A) 1
B) 3
C) 0
D) 2
E) 4
G) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 180
Related Exams