B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of CO2?
A) linear
B) trigonal planar
C) angular
D) trigonal pyramidal
E) tetrahedral
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the formula of the compound formed when ions of sodium and sulfur combine.
A) Na2S
B) NaS2
C) NaS
D) Na2S2
E) Na3S
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is TRUE concerning the solubility of a substance in H2O?
A) Polar and nonpolar substances are equally soluble in water.
B) A nonpolar substance is soluble in water.
C) All substances are soluble in water.
D) Solubility of a substance in water has no relationship to the polarity of the substance being dissolved.
E) A polar substance is soluble in water.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the ionic compound with the formula Na3PO4?
A) silver phosphite
B) sodium phosphorus oxide
C) trisilver tetraphosphate
D) trisodium tetraphosphate
E) sodium phosphate
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What kind of bond results when electron transfer occurs between atoms of two different elements?
A) ionic
B) covalent
C) nonpolar
D) single
E) double
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ionic compound magnesium hydroxide can be found in antacid tablets.What is the formula of magnesium hydroxide?
A) Mg2OH
B) MgOH2
C) MgO2H2
D) Mg(OH) 2
E) MgOH
G) C) and E)
Correct Answer

verified
Correct Answer
verified
True/False
As a rule,ionic compounds tend to have lower melting and boiling points than covalent compounds consisting of small molecules.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In what region on the periodic table are elements with the lowest electronegativity values found?
A) upper right corner
B) bottom right corner
C) upper left corner
D) bottom left corner
E) middle
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain compound has a very high melting point,and when it dissolves in water the solution conducts electricity.Which one of the following compounds would have these properties?
A) O2
B) Cr
C) CCl4
D) Na2SO4
E) He
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What kind of bonding exists in substances that consist of discrete molecules?
A) ionic
B) covalent
C) hydrogen bonding
D) intermolecular
E) polar
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is NOT true about elements that form cations?
A) The atoms lose electrons in forming ions.
B) The elements are metals.
C) They are located to the left of the periodic table.
D) They have low ionization energies.
E) They have high electron affinities.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the VSEPR theory,what is the geometry (shape) around the phosphorus atom in the Lewis structure shown below? 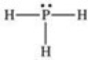
A) linear
B) bent (angular)
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 93 of 93
Related Exams