A) the product concentration does not change significantly
B) the substrate concentration is large and does not change significantly
C) the concentration of enzyme-substrate complex remains constant with time
D) the free enzyme concentration is always in great excess to the concentration of enzyme-substrate complex
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following diseases has not been successfully treated using the principles of enzyme inhibition?
A) AIDS.
B) Lactose intolerance
C) Virus infection
D) Neither AIDS nor virus infection.
E) All of these have been successfully treated using enzyme inhibitors.
G) None of the above
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:  The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: 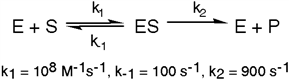 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. "Hindrate" is an inhibitor of triose phosphate isomerase. When it is added to cells at a concentration of 0.1 nM, the enzyme's KM for the substrate is unchanged, but the apparent Vmax is altered to 50 nM\sec.
In the following graph, which line best represents the Lineweaver-Burk plot obtained in the presence of hindrate?
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. "Hindrate" is an inhibitor of triose phosphate isomerase. When it is added to cells at a concentration of 0.1 nM, the enzyme's KM for the substrate is unchanged, but the apparent Vmax is altered to 50 nM\sec.
In the following graph, which line best represents the Lineweaver-Burk plot obtained in the presence of hindrate? 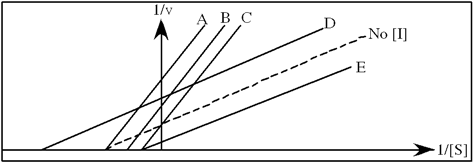
A) A
B) B
C) C
D) D
E) E
G) C) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following is true about a mixed type inhibition?
A) A Lineweaver-Burk plot will give parallel lines
B) The KM will change but not the Vmax
C) The lines of a Lineweaver-Burk graph will cross in the top left quadrant
D) None of these is true
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The active site of an enzyme
A) is frequently located in a cleft in the enzyme.
B) is the portion of the enzyme to which the substrate binds.
C) contains the reactive groups that catalyze the reaction.
D) all of these are correct
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true?
A) The E-S complex often dissociates with no reaction taking place.
B) The E-S complex must form before a reaction can take place
C) Once the E-S complex forms, it can go on to form product or dissociate to E + S
D) All of these are correct
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A noncompetitive inhibitor
A) binds to the enzyme at a site other than the active site
B) is structurally related to the substrate
C) does not affect the value of Vmax
D) decreases the value of KM
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the induced-fit model of substrate binding to enzymes
A) the substrate changes its conformation to fit the active site
B) the active site changes its conformation to fit the substrate
C) there is a conformational change in the enzyme when the substrate binds
D) there is aggregation of several enzyme molecules when the substrate binds
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true about the enzyme chymotrypsin?
A) The enzyme can cleave peptides.
B) The enzyme can cleave esters.
C) The enzyme only binds to aromatic substrates.
D) The enzyme can cleave substrates which are not naturally occurring.
E) All of these are correct
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
To study the nature of an enzyme, Vmax is not as good a measurement as the catalytic rate constant kcat because:
A) The Vmax is not a true constant since it depends on the concentration of enzyme
B) The Vmax cannot be measured
C) The Vmax is only valid for allosteric enzymes
D) none of these
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction catalyzed by aspartate transcarbamoylase is
A) the first step in the synthesis of amino acids.
B) the first step in the synthesis of fatty acids.
C) the first step in the synthesis of CTP and UTP.
D) is part of glycolysis.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:  The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: 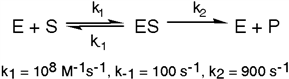 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the equilibrium constant for the uncatalyzed reaction?
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the equilibrium constant for the uncatalyzed reaction?
A) 0.9
B) 1.1
C) 2.5
D) Cannot be determined from the information provided.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:  The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: 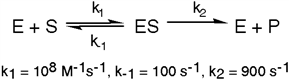 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. "Hindrate" is an inhibitor of triose phosphate isomerase. When it is added to cells at a concentration of 0.1 nM, the enzyme's KM for the substrate is unchanged, but the apparent Vmax is altered to 50 nM\sec.
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. "Hindrate" is an inhibitor of triose phosphate isomerase. When it is added to cells at a concentration of 0.1 nM, the enzyme's KM for the substrate is unchanged, but the apparent Vmax is altered to 50 nM\sec.
A) This is a competitive inhibitor.
B) This is an uncompetitive inhibitor.
C) This is a noncompetitive inhibitor.
D) This is an irreversible inhibitor.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The E-S complex often shows as a slight depression in the energy profile for the reaction.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The main difference between a catalyzed and an uncatalyzed reaction is that
A) the activation energy of the catalyzed reaction is lower.
B) the catalyzed reaction has a more favorable free energy change.
C) the catalyzed reaction has a more favorable enthalpy change.
D) the catalyzed reaction has a more favorable entropy change.
F) A) and B)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Enzymatic activity has an optimum temperature because
A) the component amino acids have varying melting points
B) the rate of reactions is thermodynamically controlled
C) the side chains of essential residues are chemically degraded at higher temperatures
D) raising the temperature speeds up the reaction until protein denaturation sets in
E) the organism dies beyond a certain temperature
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Non-competitive inhibitors have this effect:
A) Modifying the KM value.
B) Changing the value for Vmax.
C) Interfering with substrate binding.
D) This type of inhibitor both changes the Vmax and interferes with substrate binding.
E) All of these are correct.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The amount of energy released during a reaction tells nothing about the rate at which that reaction will occur.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:  The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: 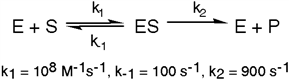 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the actual velocity of the forward reaction under physiologic conditions?
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the actual velocity of the forward reaction under physiologic conditions?
A) 2 nM\s
B) 45 nM\s
C) 500 nM\s
D) 30 nM\s
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:  The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway. It follows simple Michaelis-Menten kinetics: 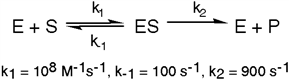 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the equilibrium constant for the enzyme-catalyzed reaction?
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A. What is the equilibrium constant for the enzyme-catalyzed reaction?
A) 0.9
B) 1.1
C) 2.5
D) Cannot be determined from the information provided.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 88
Related Exams