A) Carbon - 14 decays the fastest and is the most stable.
B) Carbon - 14 decays the fastest and Lead - 210 is the most stable.
C) Lead - 210 decays the fastest and is the most stable.
D) Lead - 210 decays the fastest and Carbon - 14 is the most stable.
E) They both decay at the same rate and Carbon - 14 is the most stable.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One of the fission reactions that occurs in uranium reactors is:
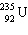 +
+ 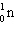
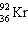 +
+  + ____ To balance this nuclear equation the blank is:
+ ____ To balance this nuclear equation the blank is:
A) "10n"
B) "3 10n"
C) "42He"
D) "31H"
E) "none of these"
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Provided in the table below are two radionuclides and their corresponding half-life periods. Which radionuclide decays the fastest and which isotope is the least stable ? Isotope Half-life period Lead - 210 22 Years Platinum - 190 6.9×1011 Years
A) Lead - 210 decays the fastest and is the least stable.
B) Lead - 210 decays the fastest and Platinum - 190 is the least stable.
C) Platinum - 190 decays the fastest and is the least stable.
D) Platinum - 190 decays the fastest and Lead - 210 is the least stable.
E) They both decay at the same rate and Lead - 210 is the least stable.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Curium - 248, 248Cm, was bombarded, yielding Antimony - 116, 116Sb, and Cesium - 160, 160Cs. What was the bombarding nucleus?
A) "7 42 a"
B) "28Ne"
C) "28Ni"
D) "28Si"
E) "10B"
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Provided in the table below are two isotopes and their corresponding half-life periods. Which isotope decays the fastest and which isotope is the most stable ? Isotope Half-life period Vanadium - 50 6.0×1015 Years Uranium - 235 7.1×108 Years
A) Vanadium - 50 decays the fastest and is the most stable.
B) Vanadium - 50 decays the fastest and Uranium - 235 is the most stable.
C) Uranium - 235 decays the fastest and is the most stable.
D) Uranium - 235 decays the fastest and Vanadium - 50 is the most stable.
E) They both decay at the same rate and Vanadium - 50 is the most stable.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which two natural radioactive decay processes will produce the same product nuclide?
A) a decay and ![]() decay
decay
B) ![]() and electron capture
and electron capture
C) ![]() and
and ![]()
D) ![]() and electron capture
and electron capture
E) none of these
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What must be placed in the blank to produce a balanced nuclear equation?
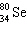 +
+ 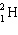 ____ +
____ + 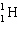
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) none of these
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider comparing the two radionuclides given below and their corresponding half-life periods. Which statement below is true? Isotope Half-life period Carbon - 14 5730 years Iodine - 131 8 days
A) Carbon - 14 decays faster and is the most stable nuclide.
B) Carbon - 14 decays faster and is the least stable nuclide.
C) Iodine - 131 decays faster and is the most stable nuclide.
D) Iodine - 131 decays faster and is the least stable nuclide.
E) They both decay at the same rate but Carbon - 14 is more stable.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction below can be best described as what type of nuclear reaction? 23592U + 10n→13956Ba + 9436Kr + 3 10n
A) Alpha decay
B) Beta decay
C) Nuclear fission
D) Nuclear fusion
E) Radioactive decay
G) None of the above
Correct Answer

verified
Correct Answer
verified
Showing 81 - 89 of 89
Related Exams