A) H+ < Na+ < Mg2+
B) Mg2+ < Ca2+ < Ba2+
C) Mg2+ < Ba2+ < Ca2+
D) Ca2+ < K+ < Rb+
E) Rb+ < K+ < Ca2+
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
________ is a measure of the degree to which the electron cloud surrounding an atom or molecule can be distorted in an electric field.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which intermolecular force or bond is responsible for the density of H2O(s) being less than that of H2O( 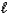 ) ?
) ?
A) London dispersion forces
B) hydrogen bonding
C) ionic bonding
D) covalent bonding
E) dipole/induced dipole forces
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
On a relative basis,the weaker the intermolecular forces in a substance are,
A) the larger is its heat of vaporization.
B) the more it deviates from the ideal gas law.
C) the greater is its vapor pressure at a particular temperature.
D) the larger is its molar heat capacity as a liquid.
E) the higher is its boiling point.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 75.0 C,water has an equilibrium vapor pressure of 289.1 mm Hg.If 4.22 g H2O is sealed in an evacuated 5.00 L flask and heated to 75.0 C,what mass of H2O will be found in the gas phase when liquid-vapor equilibrium is established? Assume any liquid remaining in the flask has a negligible volume.(R = 0.08206 L.atm/mol.K,1 atm = 760 mm Hg)
A) 0.834 g
B) 1.20 g
C) 1.92 g
D) 3.02 g
E) 4.22 g
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning induced dipole/induced dipole forces is/are CORRECT? 1) In general,induced dipole/induced dipole interactions decrease as the size of a molecule increases. 2) Induced dipole/induced dipole forces are the attractive forces in molecular solids consisting of nonpolar molecules. 3) Induced dipole/induced dipole forces exist in both polar and nonpolar molecular solids.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a water molecule forms a hydrogen bond with another water molecule,which atoms are involved in the interaction?
A) a hydrogen from one molecule and a hydrogen from the other molecule
B) a hydrogen from one molecule and an oxygen from the other molecule
C) an oxygen from one molecule and an oxygen from the other molecule
D) an oxygen and a hydrogen from the same molecule
E) two hydrogens from one molecule and one hydrogen from the other molecule
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A liquid has an enthalpy of vaporization of 30.4 kJ/mol.At 269 K it has a vapor pressure of 102 mmHg.What is the normal boiling point of this liquid? (R = 8.314 J/(K· mol) )
A) 287 K
B) 316 K
C) 269 K
D) 253 K
E) 234 K
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The heat of vaporization of benzene,C6H6,is 30.7 kJ/mol at its boiling point of 80.1 C.How much energy in the form of heat is required to vaporize 148 g benzene at its boiling point?
A) 0.207 kJ
B) 4.82 kJ
C) 16.2 kJ
D) 58.2 kJ
E) ![]() kJ
kJ
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule will be polar if it ___.
A) has polar bonds
B) contains both carbon and chlorine
C) consists of more than three atoms
D) is diatomic with different electronegativities
E) contains atoms with different electronegativities
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a particular liquid,raising its temperature from 321 K to 352 K causes its vapor pressure to double.What is the enthalpy of vaporization of this liquid? (R = 8.314 J/K · mol)
A) 21.0 kJ/mol
B) 186 kJ/mol
C) 3.29 kJ/mol
D) 383 kJ/mol
E) 179 kJ/mol
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
The ________ equation relates the equilibrium vapor pressure of a volatile liquid to the molar enthalpy of vaporization at a given temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 3.21 g of water is sealed in an evacuated 2.52 L flask and heated to the normal boiling point of 373 K,what is the pressure in the flask? (R = 0.08206 L.atm/mol.K)
A) 0.231 atm
B) 0.462 atm
C) 1.00 atm
D) 2.16 atm
E) 39.0 atm
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Freon-113,C2Cl3F3,has an enthalpy of vaporization of 27.0 kJ/mol and a normal boiling point of 48.0 C.What is the vapor pressure (in atm) of Freon-113 at 22.0 C? (R = 8.314 J/K.mol)
A) 0.21 atm
B) 0.35 atm
C) 0.41 atm
D) 0.46 atm
E) 4.4 atm
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has a boiling point closest to that of argon?
A) F2
B) Cl2
C) HCl
D) NaF
E) HF
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What intermolecular force or bond is primarily responsible for the solubility of CH3OH in water?
A) ion-dipole force
B) dipole-dipole force
C) ionic bonding
D) covalent bonding
E) hydrogen bonding
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning intermolecular forces is/are CORRECT? 1) Dipole-dipole attractions occur in all molecules that contain polar bonds,regardless of whether the molecule has a dipole. 2) Induced dipole/induced dipole forces exist in all molecular solids. 3) Hydrogen bonding only occurs in all molecules containing OH bonds.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
List all the intermolecular forces present in pure acetone. 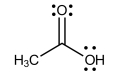
A) hydrogen bonding only
B) dipole-dipole force only
C) dipole-dipole force and London dispersion forces
D) hydrogen bonding and London dispersion forces
E) hydrogen bonding,dipole-dipole force,and London dispersion forces
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sulfur dioxide has a vapor pressure of 462.7 mm Hg at -21.0 C and a vapor pressure of 140.5 mm Hg at -44.0 C.What is the enthalpy of vaporization of sulfur dioxide? (R = 8.314 J/K.mol)
A) 0.398 kJ/mol
B) 6.33 kJ/mol
C) 14.0 kJ/mol
D) 24.9 kJ/mol
E) 39.8 kJ/mol
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 79 of 79
Related Exams