A) 0.048 M/s
B) 0.016 M/s
C) 0.144 M/s
D) 0.072 M/s
E) 0.032 M/s
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Indicate which of the following compounds is a component of photochemical smog.
A) H2O
B) CO2
C) N2O
D) O3
E) CO
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One reaction that occurs in an automobile catalytic converter is the conversion of carbon monoxide to carbon dioxide. How is the rate of this reaction related to the rate at which the concentration of a reactant or product changes? 2CO(g) O2(g) →2CO2(g)
I. Rate 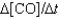 II. Rate
II. Rate 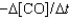 III. Rate
III. Rate 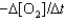 IV. Rate
IV. Rate 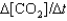
A) I only
B) II only
C) III only
D) II and III only
E) II, III, and IV only
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A scientist conducts an experiment to determine the rate of the following reaction: 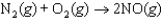 If the initial concentration of NO was 0.00 M and the concentration of NO was 0.050 M after 0.100 s, what is the average rate of the reaction?
If the initial concentration of NO was 0.00 M and the concentration of NO was 0.050 M after 0.100 s, what is the average rate of the reaction?
A) 0.500 M/s
B) 1.00 M/s
C) 5.00 M/s
D) 10.0 M/s
E) 0.250 M/s
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following data for the reaction A  B, determine the frequency factor, A, of the reaction. k (M/s)
T (K)
2) 04 104
250
6) 78 103
400
B, determine the frequency factor, A, of the reaction. k (M/s)
T (K)
2) 04 104
250
6) 78 103
400
A) 2.3
B) 5.3
C) 0.99
D) 0.63
E) 0.85
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate law of a particular reaction was determined to be Rate k[A][B]. What is the unit of the rate constant for the reaction?
A) M/s
B) 1/(Ms)
C) 1/(Ms2)
D) 1/s
E) Ms
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mechanism for the first-order reaction 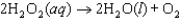 in the presence of I(aq) is proposed to be: Step 1:
in the presence of I(aq) is proposed to be: Step 1: 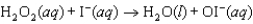 (slow)
Step 2:
(slow)
Step 2: 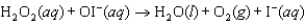 (fast)
Identify the catalyst in the reaction.
(fast)
Identify the catalyst in the reaction.
A) H2O2
B) OI
C) I
D) H2O
E) O2
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
The device in automobiles that has decreased NO and partially oxidized hydrocarbons from exhaust gases is termed a(n) ________ .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mechanism for the reaction 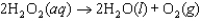 in the presence of I(aq) is proposed to be: Step 1:
in the presence of I(aq) is proposed to be: Step 1: 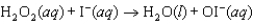 (slow)
Step 2:
(slow)
Step 2: 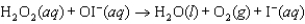 (fast)
What is the molecularity of the rate-determining step?
(fast)
What is the molecularity of the rate-determining step?
A) zero molecular
B) unimolecular
C) bimolecular
D) termolecular
E) More information is needed to answer this question.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
N2O5 is used as a source of NO2 in chemical reactions. The compound decomposes in a first order reaction. If the initial concentration of N2O5 0.400 M, and the concentration is 0.025 M after 120 seconds, what is the rate constant k of the reaction?
A) 7.50 s1
B) 333 s1
C) 2.77 s1
D) 2.31 102 s1
E) 5.21 104 s1
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction A(g) B(g) ![The reaction A(g) <font face= symbol ></font> B(g) <font face= symbol ></font>C(g) has the following rate law: Rate <font face= symbol ></font> k[A]<sup>2</sup>[B]. If the concentration of A is tripled while the concentration of B is doubled, the rate will increase by a factor of ________. A) 9 B) 18 C) 12 D) 6 E) 24](https://d2lvgg3v3hfg70.cloudfront.net/TB3835/11eaa952_9b6b_d8b3_ae90_cf409b8d6e45_TB3835_11.jpg) C(g) has the following rate law: Rate k[A]2[B]. If the concentration of A is tripled while the concentration of B is doubled, the rate will increase by a factor of ________.
C(g) has the following rate law: Rate k[A]2[B]. If the concentration of A is tripled while the concentration of B is doubled, the rate will increase by a factor of ________.
A) 9
B) 18
C) 12
D) 6
E) 24
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Collision theory assumes that the rate of a reaction depends on ________
A) the energy of collisions.
B) the orientation of colliding molecules.
C) the energy of collisions and the orientation of colliding molecules.
D) the change in energy between the products and the reactants.
E) the change in free energy between the reactants and products.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The energy profiles for four different reactions are shown below. The scales are the same for each. Which reaction requires the least energetic collisions to reach the transition state? 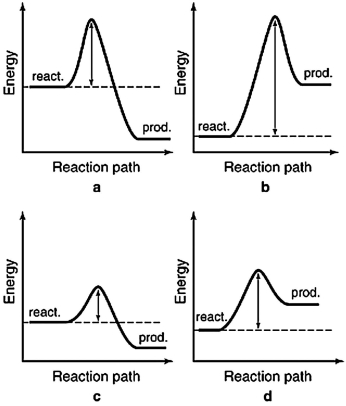
A) a
B) b
C) c
D) d
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 2A 3B  4C 5D, the rate of the reaction in terms of A would be written as ________
4C 5D, the rate of the reaction in terms of A would be written as ________
A) (![]() )
)
B) (- ![]()
![]() )
)
C) ( ![]() )
)
D) (![]()
![]() )
)
E) ( ![]() )
)
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The steps in a reaction mechanism are as follows. Which species is acting as a catalyst? Step 1: 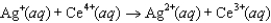 Step 2:
Step 2: 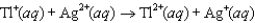 Step 3:
Step 3: 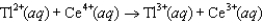
A) Ag
B) Tl
C) Ce3
D) Ag2
E) Tl3
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
NO2 contributes to the "brown haze" associated with photochemical smog events. At what time of day is NO2 concentration highest?
A) in the morning before rush hour
B) in the morning just after rush hour
C) midmorning
D) noon
E) midafternoon
G) None of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
The rate of popcorn popping at different temperatures was found to be described by the Arrhenius equation. How many times faster does the popcorn pop at 210°C compared with that at 180°C when the activation energy is 167 kJ/mol?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One reaction that occurs in an automobile catalytic converter is the conversion of nitrogen monoxide to nitrogen and oxygen. How could the rate of this reaction be expressed correctly in terms of the rate at which the concentration of a reactant or product changes? 2NO(g)  N2(g) O2(g)
N2(g) O2(g)
A) Rate ![]()
B) Rate ![]()
C) Rate ![]()
D) Rate ![]()
E) Rate ![]()
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The energy needed to form an activated complex is called ________
A) collision energy.
B) kinetic energy.
C) activation energy.
D) potential energy.
E) thermodynamic energy.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following data for the reaction A  B, determine the frequency factor, A, of the reaction.
B, determine the frequency factor, A, of the reaction. 
A) (0.75)
B) (0.719)
C) (2.05)
D) (0.287)
E) (0.287)
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 155
Related Exams